Predict the product of the following SN2 reaction. This comprehensive guide delves into the intricacies of SN2 reactions, empowering you with the knowledge to accurately predict reaction outcomes. Join us as we unravel the factors that govern nucleophilic substitution reactions, providing you with a deeper understanding of organic chemistry.
SN2 reactions, a fundamental concept in organic chemistry, play a crucial role in the synthesis of various organic compounds. Understanding the mechanism and characteristics of SN2 reactions is essential for predicting the products of these reactions accurately. This guide provides a comprehensive overview of SN2 reactions, including their stereochemistry, factors influencing their products, and practical applications.
SN2 Reaction Overview: Predict The Product Of The Following Sn2 Reaction
SN2 reactions are a type of nucleophilic substitution reaction in which a nucleophile (a species with a lone pair of electrons) attacks an electrophile (a species with a positive or partially positive charge) and replaces a leaving group.
The mechanism of an SN2 reaction is a one-step process that occurs in a concerted manner. The nucleophile and electrophile come together at the same time, and the leaving group is expelled in the opposite direction.
The key features of an SN2 reaction are as follows:
- The reaction is second-order, meaning that the rate of the reaction is proportional to the concentrations of both the nucleophile and the electrophile.
- The reaction is stereospecific, meaning that the configuration of the product is determined by the configuration of the starting materials.
- The reaction is favored by polar aprotic solvents, which help to stabilize the transition state.
Predicting the Product of an SN2 Reaction
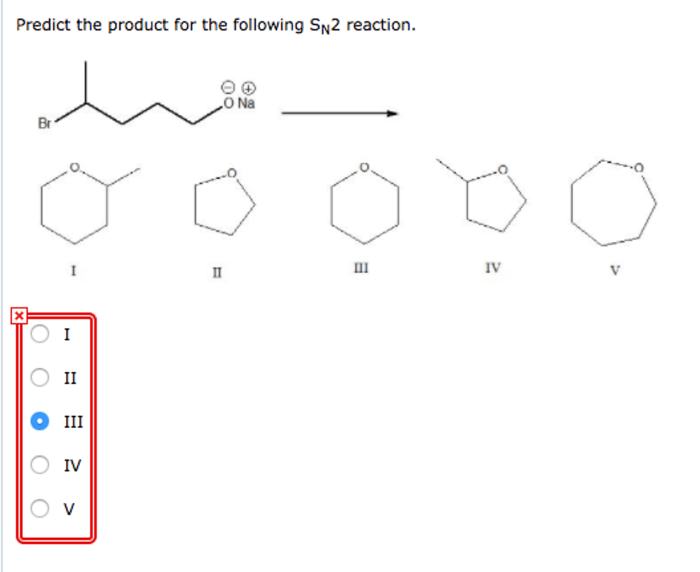
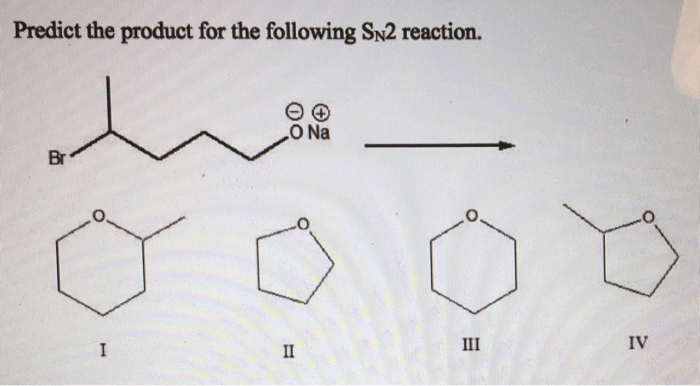
The product of an SN2 reaction can be predicted by considering the following factors:
- The nucleophilicity of the nucleophile: The more nucleophilic the nucleophile, the faster the reaction will be.
- The electrophilicity of the electrophile: The more electrophilic the electrophile, the faster the reaction will be.
- The steric hindrance around the electrophilic center: The more steric hindrance around the electrophilic center, the slower the reaction will be.
In general, the best nucleophiles are those that are small, have a high charge density, and are polarizable. The best electrophiles are those that are small, have a low charge density, and are not sterically hindered.
Stereochemistry of SN2 Reactions
SN2 reactions are stereospecific, meaning that the configuration of the product is determined by the configuration of the starting materials. In an SN2 reaction, the nucleophile attacks the electrophile from the back side, resulting in an inversion of configuration at the reaction center.
This inversion of configuration can be illustrated by the following example:
When (S)-2-bromobutane reacts with hydroxide ion in an SN2 reaction, the product is (R)-2-butanol.
Examples of SN2 Reactions
SN2 reactions are a versatile tool for the synthesis of a wide variety of organic compounds. Some examples of SN2 reactions include:
- The reaction of alkyl halides with hydroxide ion to form alcohols
- The reaction of alkyl halides with alkoxide ions to form ethers
- The reaction of alkyl halides with amines to form amines
The rate and selectivity of SN2 reactions can be influenced by a number of factors, including the solvent, the temperature, and the presence of catalysts.
Applications of SN2 Reactions

SN2 reactions are used in a variety of organic synthesis applications, including:
- The preparation of alcohols
- The preparation of ethers
- The preparation of amines
- The synthesis of complex organic molecules
SN2 reactions are a powerful tool for the synthesis of a wide variety of organic compounds. By understanding the factors that influence the product and stereochemistry of SN2 reactions, chemists can use these reactions to efficiently synthesize the desired products.
Clarifying Questions
What is an SN2 reaction?
An SN2 reaction is a nucleophilic substitution reaction in which a nucleophile attacks an electrophile, resulting in the inversion of the configuration at the reaction center.
What factors influence the product of an SN2 reaction?
The product of an SN2 reaction is influenced by the nucleophilicity of the nucleophile, the electrophilicity of the electrophile, and the steric hindrance around the reaction center.
What is the stereochemistry of an SN2 reaction?
SN2 reactions proceed with inversion of configuration at the reaction center, meaning that the product has the opposite stereochemistry compared to the starting material.