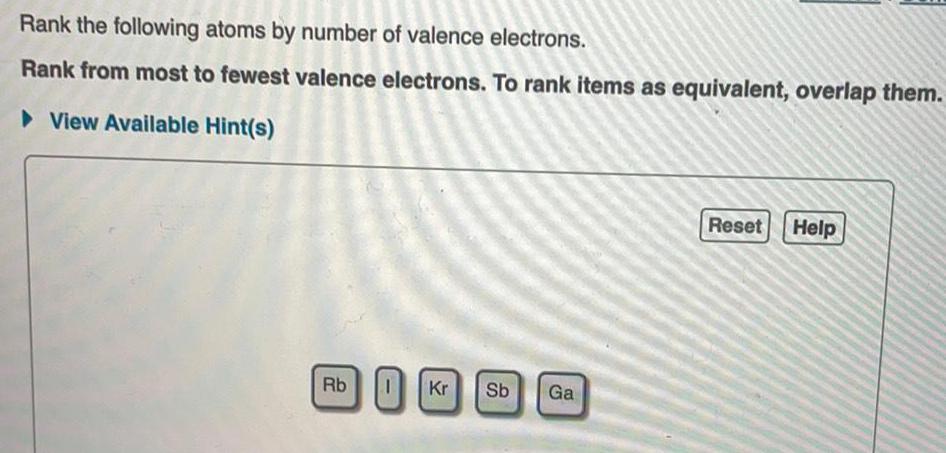
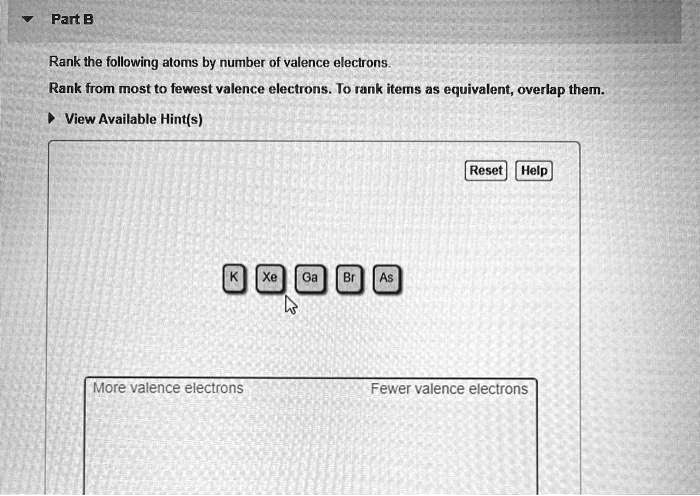
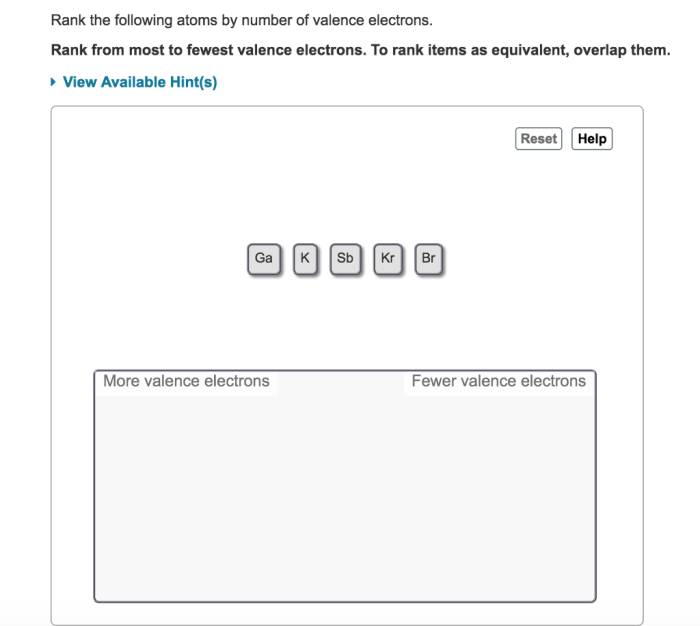
Rank the following atoms by number of valence electrons. This fundamental concept in chemistry plays a pivotal role in determining the chemical properties of atoms and their ability to form bonds. Understanding valence electrons is crucial for comprehending the behavior of atoms and their interactions with each other.
Delving into the intricacies of valence electrons, this guide will elucidate their significance, provide a step-by-step approach to ranking atoms based on their valence electron count, and explore the practical applications of this knowledge in various scientific disciplines.
Valence Electrons in Atoms: Rank The Following Atoms By Number Of Valence Electrons.
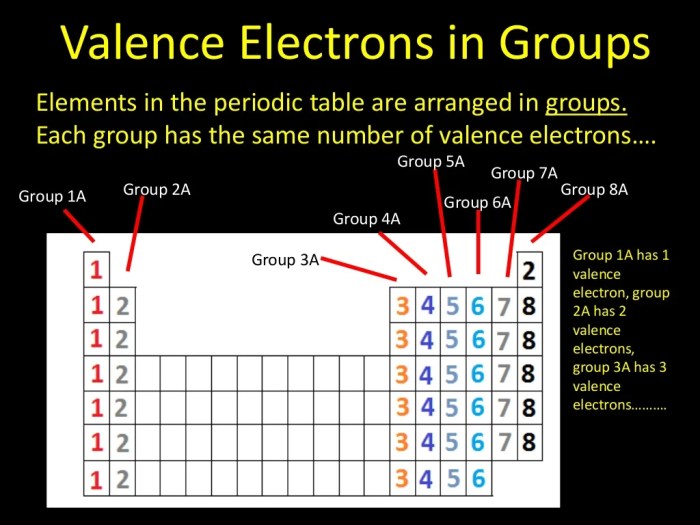
Valence electrons are the electrons in the outermost electron shell of an atom. They are the electrons that participate in chemical bonding and determine the chemical properties of an atom.
The number of valence electrons in an atom can be determined by looking at the atom’s position in the periodic table. The elements in the first column of the periodic table have one valence electron, the elements in the second column have two valence electrons, and so on.
The elements in the last column of the periodic table, known as the noble gases, have a full valence shell and are therefore unreactive.
Ranking Atoms by Valence Electrons
Atoms can be ranked by the number of valence electrons they have. The atoms with the most valence electrons are the most reactive, while the atoms with the fewest valence electrons are the least reactive.
The following steps can be used to rank atoms by the number of valence electrons they have:
- Identify the element’s position in the periodic table.
- Determine the number of valence electrons the element has based on its position in the periodic table.
- Rank the atoms from the most valence electrons to the least valence electrons.
The significance of valence electrons in determining the chemical properties of atoms is that the number of valence electrons determines the type of chemical bonds that an atom can form.
Examples of Ranked Atoms
The following table shows a list of atoms ranked by the number of valence electrons they have:
| Atom | Atomic Number | Number of Valence Electrons |
|---|---|---|
| Hydrogen | 1 | 1 |
| Helium | 2 | 2 |
| Lithium | 3 | 1 |
| Beryllium | 4 | 2 |
| Boron | 5 | 3 |
| Carbon | 6 | 4 |
| Nitrogen | 7 | 5 |
| Oxygen | 8 | 6 |
| Fluorine | 9 | 7 |
| Neon | 10 | 8 |
Applications of Valence Electron Ranking, Rank the following atoms by number of valence electrons.
Ranking atoms by valence electrons has a number of practical applications in chemistry and other fields.
In chemistry, valence electron ranking can be used to:
- Predict the chemical properties of an element.
- Determine the type of chemical bonds that an atom can form.
- Design new materials with specific properties.
In other fields, valence electron ranking can be used to:
- Understand the electronic structure of materials.
- Develop new electronic devices.
- Predict the reactivity of molecules.
Question & Answer Hub
What are valence electrons?
Valence electrons are the electrons in the outermost energy level of an atom, which determine its chemical properties and ability to form bonds.
Why is ranking atoms by valence electrons important?
Ranking atoms by valence electrons helps predict their chemical behavior, as atoms with similar valence electron counts tend to exhibit similar chemical properties.
How do valence electrons influence chemical bonding?
Valence electrons are responsible for forming chemical bonds by interacting with valence electrons from other atoms, leading to the formation of molecules and compounds.